01
Ethylene oxide sterilization and desorption
The ethylene oxide sterilization process is a crucial step in the production of medical masks. During the production process, a large number of masks are sent into the disinfection room, and then ethylene oxide gas is introduced. Once it reaches a specific concentration, the disinfection process is completed. However, this does not mean that masks can leave the factory immediately, as there will be residual ethylene oxide on them. Ethylene oxide is a toxic and carcinogenic substance. Long-term inhalation not only irritates the respiratory tract but may also cause cancer. Therefore, masks sterilized with ethylene oxide must undergo a desorption process to ensure that the residual ethylene oxide meets safety standards. This process usually takes 7 to 15 days to ensure that the ethylene oxide content in the mask is below 10ug/g. In addition, choosing breathable inner packaging materials is crucial for ensuring the sterilization effect, as ethylene oxide needs to be able to penetrate the packaging material to achieve the sterilization purpose.
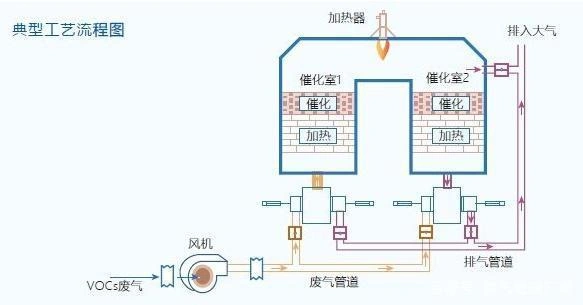
Ethylene oxide sterilization process
While understanding ethylene oxide sterilization, we also need to pay attention to its tail gas treatment methods. Common methods include direct combustion and absorption. The direct combustion method uses auxiliary fuels such as gas or oil to burn ethylene oxide, causing it to decompose into non-toxic substances at high temperatures. However, this method poses an explosion risk, requires strict control of the concentration of ethylene oxide, and demands high safety technology and operational proficiency. The absorption method uses physical means to introduce ethylene oxide waste gas into the absorption liquid for purification. After the absorption liquid becomes saturated, the waste gas is treated through heating, desorption, condensation and recovery. This method is suitable for treating ethylene oxide waste gas with large volume, low concentration and low temperature.
Analysis process and selection of packaging materials
The analysis process takes 7 to 15 days to ensure that the ethylene oxide content in the mask is below 10ug/g. The selection of breathable packaging materials is crucial for ensuring the sterilization effect.
Exhaust gas treatment methods
Direct combustion method
Ethylene oxide is decomposed into non-toxic substances through high temperatures, but this method poses an explosion risk and requires high safety technology and operational proficiency.

Absorption process
The purification of ethylene oxide waste gas by physical methods requires heating, desorption, condensation and recovery, which involves large equipment investment and high treatment difficulty.
Catalytic combustion method
Ethylene oxide is heated to burn and convert into non-toxic CO2 and H2O under the action of a catalyst. This method has the advantages of low ignition temperature, energy conservation and high purification rate, but it occupies a relatively large area and requires relatively large equipment investment.
Adsorption
The adsorption of ethylene oxide by adsorbents such as activated carbon is simple in equipment and requires a relatively small investment. However, activated carbon needs to be replaced regularly, which may increase the volume of hazardous waste to be treated.
Adsorption-catalytic combustion method
Ethylene oxide is first adsorbed by adsorption materials such as activated carbon. When the adsorption is close to saturation, hot air is introduced for desorption and desorption. The ethylene oxide waste gas after analysis is then introduced into the catalytic combustion bed for thorough purification. This method can reduce the generation of hazardous waste and lower energy consumption, but it also occupies a large area and requires a relatively large investment in equipment.
Atmospheric pressure catalytic hydration method
Under acidic conditions, the hydration reaction of ethylene oxide aqueous solution with dilute sulfuric acid as a catalyst is carried out to produce ethylene glycol (EG). This method operates under normal pressure and the catalyst is relatively inexpensive. However, it should be noted that inorganic acid catalysts may cause corrosion to carbon steel equipment, while inorganic alkali catalysts may produce by-products.
Direct hydration method
This method does not explicitly mention specific details in the text. You can learn about it based on your actual situation. The direct hydration method, also known as the ethylene oxide pressurized hydration method, is a technique that involves reacting water and ethylene oxide in a tubular reactor at a specific molar ratio. Under conditions of 150 to 200 ° C and 15 to 2.5MPa, the reaction lasted for 20 minutes, thereby directly obtaining ethylene glycol (EG) through liquid-phase hydration. However, this process also generates by-products, such as Diethylene Glycol (DEG for short), Triethylene Glycol (TEG for short), and polyethylene glycol. After the reaction is completed, the EG dilute solution cooled through the heat exchanger enters the expander, where volatile components such as crotonaldehyde and acetaldehyde are blown out. Subsequently, the liquid flows into the storage tank and is pumped to the refining and evaporation process. The evaporated liquid enters the first distillation column for vacuum distillation to remove moisture. The crude EG at the top of the column then enters the second distillation column. Eventually, pure EG is obtained at the top of the column, while the components at the bottom enter the packed column to obtain various components.
The advantage of this method lies in its high conversion rate, but it also has some disadvantages, such as an increase in by-products, an increase in moisture content in ethylene glycol, and a rise in energy consumption. In addition, there are two methods available for selection: homogeneous catalytic hydration and heterogeneous catalytic hydration. Both of these methods require the use of appropriate catalysts to promote the reaction.


 19-Aug--2025
19-Aug--2025