In recent years, global attention to personal protective equipment (PPE) has reshaped how healthcare systems, distributors, and procurement professionals evaluate respiratory protection. While both disposable medical face masks and respirators such as N95 or FFP2 are widely used, they are designed for different purposes and risk environments. Understanding the difference between these two types of products is essential for making informed purchasing and usage decisions in 2026.
What Is a Disposable Medical Face Mask?
A disposable medical face mask, also commonly referred to as a surgical mask, is designed primarily to reduce the transmission of large respiratory droplets, splashes, and sprays. It also helps limit the spread of respiratory emissions from the wearer to others.
Medical masks are typically made from non-woven polypropylene materials and are constructed in multiple layers. A standard design includes an outer fluid-resistant layer, a middle filtration layer, and an inner layer that absorbs moisture from breathing. These masks are intended for single use and are widely used in hospitals, clinics, dental offices, laboratories, and general hygiene settings.
In routine healthcare environments, medical masks play a critical role in infection control. They are easy to use, comfortable for extended wear, cost-effective for large-scale procurement, and suitable for situations where protection against splashes and droplets is required rather than airborne particles.
What Is a Respirator?
Respirators, such as N95, KN95, or FFP2 masks, are designed to achieve a close facial fit and provide a higher level of filtration efficiency against airborne particles. Unlike medical masks, respirators are tested to filter a specified percentage of very small particles under standardized conditions.
Respirators are commonly used in higher-risk environments, including settings with potential exposure to airborne pathogens, fine particulates, or hazardous aerosols. They are subject to more stringent certification and fit requirements, depending on the regulatory system of each country.
Because respirators must form a tight seal around the face, they are generally less comfortable for long-term wear and require user training or fit testing in professional settings.
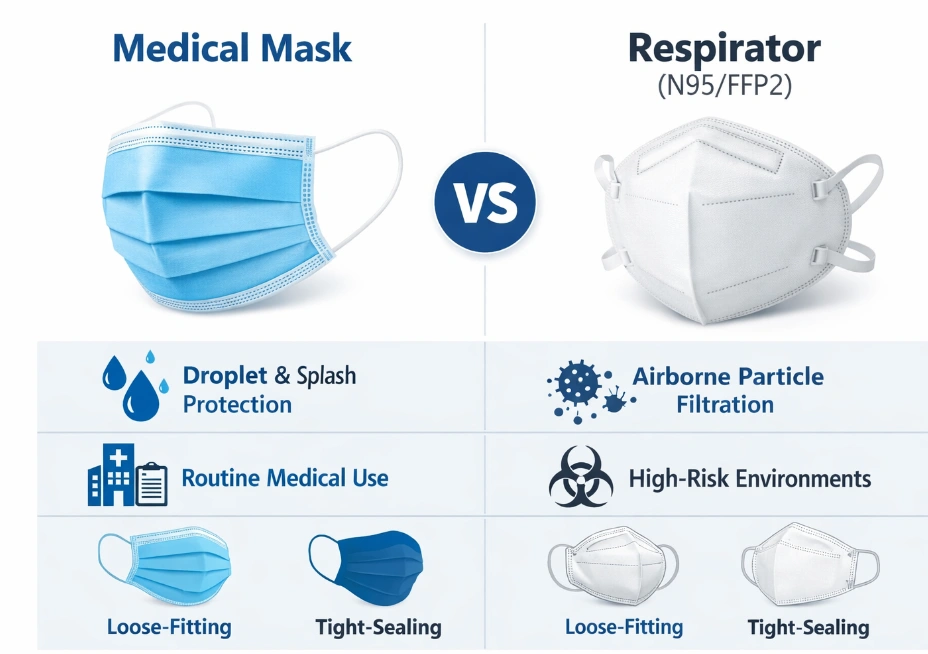
Key Differences Between Medical Masks and Respirators
Although medical masks and respirators may appear similar at first glance, their functional differences are significant:
Purpose: Medical masks focus on droplet and splash protection, while respirators provide airborne particle filtration.
Fit: Medical masks are loose-fitting, whereas respirators are designed to seal tightly against the face.
Filtration Standards: Medical masks are tested for bacterial filtration efficiency, breathability, and fluid resistance. Respirators are tested for particle filtration efficiency and fit performance.
Usage Scenarios: Medical masks are commonly used in routine healthcare and hygiene settings, while respirators are reserved for higher-risk environments.
Understanding these distinctions helps procurement teams select appropriate products based on actual risk assessments rather than assumptions.
Why Medical Masks Remain Essential in 2026
Despite ongoing discussions about airborne transmission and respirator use, disposable medical face masks continue to be an essential component of healthcare systems worldwide. Not all medical or hygiene situations require respirator-level protection, and overuse of respirators can lead to unnecessary cost increases, supply strain, and reduced wearer comfort.
Medical masks remain the preferred option for:
General patient care
Outpatient services
Dental and laboratory procedures with splash risk
Public health preparedness and stockpiling
Large-scale institutional use
Their balance of protection, comfort, and affordability makes them a foundational product in medical supply chains.
Combined Use for Enhanced Protection
In many professional environments, medical masks are used in combination with other protective equipment such as disposable face shields. Face shields provide an additional barrier against splashes and help protect the eyes and facial area, while the medical mask continues to manage respiratory droplets.
This layered approach to protection is widely recognized as an effective strategy in healthcare and industrial hygiene settings.
Considerations for Buyers and Importers
For buyers sourcing disposable medical masks or respirators in 2026, several factors should be carefully evaluated:
Compliance with applicable standards and regulations
Consistency in materials and manufacturing quality
Reliable production capacity and lead times
Clear labeling, packaging, and documentation
Suitability for the intended application
Selecting the correct product based on actual usage requirements not only improves safety but also supports more efficient procurement strategies.
Conclusion
Medical masks and respirators serve different but complementary roles in modern healthcare and protective equipment systems. Understanding their differences allows healthcare providers, distributors, and importers to make responsible, cost-effective, and compliant choices.
As global standards evolve and awareness continues to grow, disposable medical face masks will remain a core component of infection control and hygiene practices, while respirators will continue to serve specialized, high-risk applications. Choosing the right product for the right purpose is the key to effective protection in 2026 and beyond.


 04-Feb--2026
04-Feb--2026